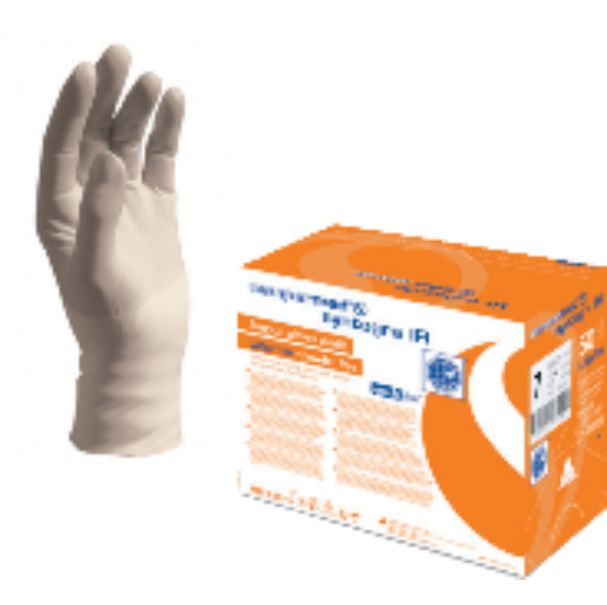
Description
Reduced allergy risk:
innovative accelerators, latex-free
Fast & easy to don:
Quick DonTM inner coating
Great comfort:
extraordinary latex-like fit & feel
Anatomical shape:
reduces strain for wearer
TYPE: Sterile surgical glove for single use, powder-free, with synthetic lining
COLOUR/ MATERIAL: Cream, Synthetic polyisoprene
GLOVE SHAPE: Fully anatomical with curved fingers and rolled rim
SIZE/OVERALL LENGTH
as per EN 455-2: 5½, 6 and 6½ min. 270mm
7, 7½ and 8 min. 280mm
8½ and 9 min. 285mm
WALL THICKNESS measured in
single layer in the palm area: 0.19–0.24mm
BARRIER PERFORMANCE: AQL 0.65, Tightness exceeding EN 455-1
(AQL 0.65 instead of AQL 1.5)
FORCE AT BREAK: ≥9N, as per EN 455-2
DURABILITY: 3 years in original packaging if stored as per DIN 7716, ISO 2230
STERILISATION: Radiation
PURPOSE
as set out in MD Directive 93/42/EEC as set out in PPE Regulation (EU) 2016/425: Medical device class IIa Single-use protective glove – PPE category III (protection against chemical substances for limited time)2)
PACKAGING: Left and right glove turned up cuff in inner pouch, ozone-tight, sealed in flat medical peel pack. In dispenser carton with sterilisation indicator: 50 pairs.
In transport carton with sterilisation indicator: 300 pairs.